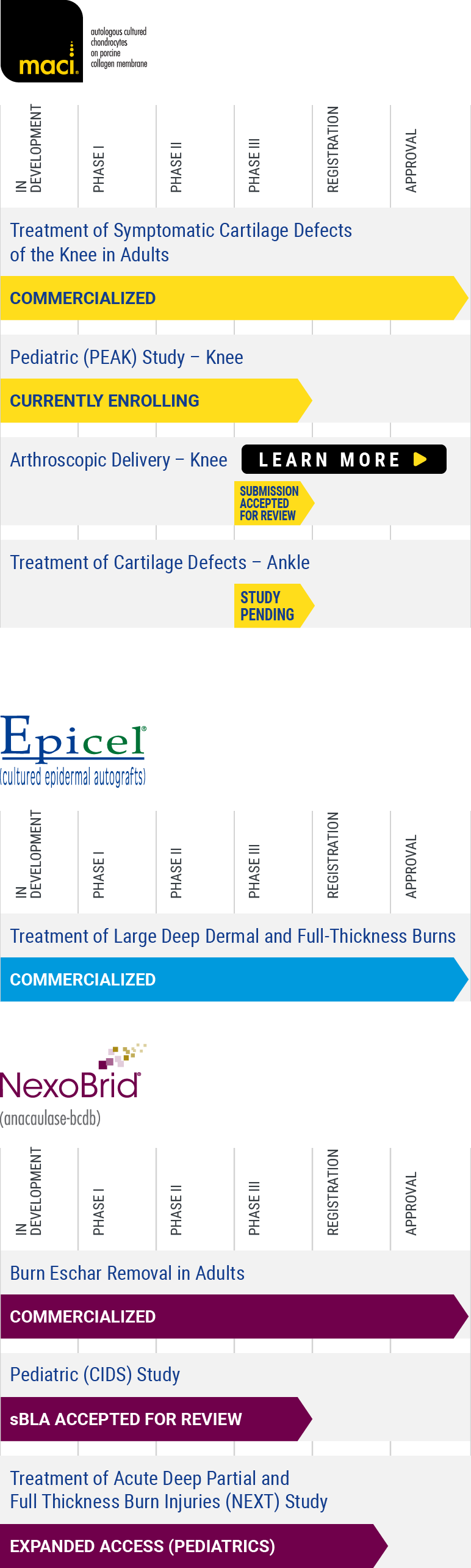
Clinical Trials
Cartilage Repair: Peak
Now recruiting for Clinical Trial PEAK (55-1702-1)
PEdiatric Autologous cultured chondrocytes treatment of cartilage defects in the Knee: A Study of MACI in Patients Aged 10 to ≤17 Years With Symptomatic Chondral or Osteochondral Defects of the Knee (PEAK).
Targets in Clinical Development: cartilage and osteochondral defects of the knee in Children and Adolescents
Cartilage and osteochondral defects of the knee occur along a spectrum of disease and severity. At one end of the spectrum are small, acute lesions that are often diagnosed incidentally at the time of knee arthroscopy and are not necessarily initially symptomatic. At the other end are larger lesions that are often symptomatic. These lesions can cause disabling symptoms such as pain, dysfunction, catching, locking, and swelling. Focal chondral lesions left untreated may progress to debilitating joint pain, dysfunction, and osteoarthritis.
The vast majority of chondral lesions occur in the adult population compared to children. In children, articular cartilage injuries that do not heal properly can result in long-term morbidity and can consume considerable healthcare resources. As in adults, cartilage injuries in the pediatric population result from various conditions. The most common pediatric cartilage injuries are associated with trauma and osteochondritis dissecans (OCD).
To learn more about clinical trials, visit ClinicalTrials.gov, a registry of federally and privately supported clinical trials conducted in the United States and around the world, or contact us with questions about our clinical development process.

PEAK (55-1702-1)
| Status: | Recruiting |
|---|---|
| Study Design: | Prospective multicenter, randomized, open-label active controlled clinical trial |
| Patients: | Patients aged 10 to ≤17 years with symptomatic articular chondral or osteochondral defects of the knee. |
| Purpose: | Compare the efficacy and safety of MACI® vs arthroscopic microfracture. |
| Publications Based On Study: | None |
You may also view more complete information about this study (55-1702-1) on ClinicalTrials.gov, a registry of clinical trials conducted in the United States and around the world. Read the full study here >
Severe Burn: CIDS
Now recruiting for Clinical Trial CIDS (NCT02278718)
A Study to Evaluate the Efficacy and Safety of NexoBrid in Children With Thermal Burns Compared to the Standard of Care
Treatment of burns depends on the severity of the burn and the burn cause (etiology); for very severe burn, which results in dead tissue (eschar), debridement, or the removal of the eschar is one of the first and important steps of burn care.
This study will be a two-arm study intending to demonstrate superiority of NexoBrid treatment over SOC in children with thermal burns. The study objective is to evaluate the safety and clinical benefit of NexoBrid in hospitalized children (0-17 years) with deep partial and/or full thickness thermal burns of 1-30% TBSA and to compare NexoBrid to standard of care (SOC).
To learn more about clinical trials, visit ClinicalTrials.gov, a registry of federally and privately supported clinical trials conducted in the United States and around the world, or contact us with questions about our clinical development process.
CIDS (NCT02278718)
| Status: | Recruiting |
|---|---|
| Study Design: | Multicenter, Multinational, Randomized, Controlled, Open Label Study |
| Patients: | Pediatric patients with thermal burns caused by fire/flame, scalds or contact with a total burns area ≥1% DPT and/or FT and ≤30% TBSA; SPT, DPT and/or FT in depth |
| Purpose: | Demonstrate superiority over standard of care for eschar removal |
| Publications Based On Study: | Rosenberg L, Krieger Y, Bogdanov-Berezovski A, Silberstein E, Shohom Y, Singer AJ. A novel rapid and selective enzymatic debridement agent for burn wound management: a multi-center RCT. Burns. 2014 May;40(3):466-74. |
You may also view more complete information about this study (NCT02278718) on ClinicalTrials.gov, a registry of clinical trials conducted in the United States and around the world. Read the full study here >
Clinical & Scientific Publications
Cartilage Repair
MACI Pivotal Clinical Studies: Journal Articles and Abstracts
Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Five-Year Follow-up of a Prospective Randomized Trial. Brittberg M, Recker D, Ilgenfritz J, Saris D on behalf of the SUMMIT Extension Study Group. Am J Sports Med. 2018;46(6):1343-1351.
Matrix-Applied Characterized Autologous Cultured Chondrocytes Versus Microfracture: Two-Year Follow-up of a Prospective Randomized Trial. Saris D, Price A, Brittberg M on behalf of the SUMMIT study group. Am J Sports Med. 2014;42(6):1384-94.
SUMMIT Extension: Matrix-induced autologous chondrocyte implant vs microfracture at three years. Saris D, Price A, Yu Q, Kili S, Brittberg M. American Academy of Orthopaedic Surgeons 2015 Annual Meeting, March 24-28.
MACI Preclinical and Histology Studies
Matrix-Induced Autologous Chondrocyte Implantation (MACI) Using a Cell-Seeded Collagen Membrane Improves Cartilage Healing in the Equine Model. Alan J. Nixon, BVSc, MS, Holly D. Sparks, DVM, Laila Begum, PhD, Sean McDonough, DVM, PhD, Michael S. Scimeca, MS, Nance Moran, PhD, and Gloria L. Matthews, DVM, PhD. Journal of Bone and Joint Surgery. 2017; 99(23):1987-1998
Matrix-induced autologous chondrocyte implantation (MACI) biological and histological assessment. Zheng MH, Willers C, Kirilak I, Yates P, Xu J, Wood D, Shimmin A. Tissue Eng. 2007;13:737-746.
Rehabilitation Studies
A Prospective, Randomized Comparison of Traditional and Accelerated Approaches to Postoperative Rehabilitation following Autologous Chondrocyte Implantation: 2-Year Clinical Outcomes. Ebert JR, Robertson WB, Lloyd DG, Zheng MH, Wood DJ, Ackland T. Cartilage. 2010;1(3):180-187.
Clinical and magnetic resonance imaging-based outcomes to 5 years after matrix-induced autologous chondrocyte implantation to address articular cartilage defects in the knee. Ebert JR, Robertson WB, Woodhouse J, Fallon M, Zheng MH, Ackland T, Wood DJ. Am J Sports Med. 2011;39(4):753-63.
A randomized trial comparing accelerated and traditional approaches to postoperative weightbearing rehabilitation after matrix-induced autologous chondrocyte implantation: findings at 5 years. Ebert JR, Fallon M, Zheng MH, Wood D, Ackland T. Am J Sports Med. 2012;40(7):1527-1537.
Accelerated weightbearing rehabilitation after MACI in the tibiofemoral joint. Edwards PK, Ackland T, Ebert J. Am J Sports Med. 2013;41(10):2314-24.
Two-year outcomes of a randomized trial investigating a 6-week return to full weightbearing after matrix-induced autologous chondrocyte implantation. Ebert JR, Edwards PK, Fallon M, Ackland TR, Janes GC, Wood DJ. Am J Sports Med. 2017;45(4):838-848.
Is delayed weightbearing after MACI in the knee associated with better outcomes? A systemic review of Level 1 Evidence. Kraeutler M, Belk J, Carver T, McCarty E. Orthop J Sports Med. 2018;6(5). DOI: 10.1177/2325967118770986.
Consensus on rehabilitation guidelines among orthopedic surgeons in the United States following use of third-generation articular cartilage repair (MACI) for treatment of knee cartilage lesions. Flanigan DC, Sherman SL, Chilelli B, Gersoff W, Jones D, Lee CA, Toth A, Cramer C, Zaporojan V, Carey J. Cartilage 2020. DOI: https://doi.org/10.1177/1947603520968876
MACI Treatment for Patellofemoral Defects
10-Year Prospective Clinical and Radiological Evaluation After Matrix-Induced Autologous Chondrocyte Implantation and Comparison of Tibiofemoral and Patellofemoral Graft Outcomes. Ebert, J. R., Zheng, M., Fallon, M., Wood, D. J., & Janes, G. C. (2024). The American Journal of Sports Medicine. (First published online February 21, 2024).
A Comparison of 2-year Outcomes in Patients Undergoing Tibiofemoral or Patellofemoral Matrix-Induced Autologous Chondrocyte Implantation. Ebert JR, Schneider A, Fallon M, Wood DJ, Janes GC. Am J Sports Med. 2017; 45(14): 3243-3253.
Prospective clinical and radiologic evaluation of patellofemoral matrix-induced autologous chondrocyte implantation. Ebert JR, Fallon M, Smith A, Janes GC, Wood DJ. Am J Sports Med. 2015;43(6):1362-72.
Matrix-induced autologous chondrocyte implantation (MACI) for chondral defects in the patellofemoral joint. Meyerkort D, Ebert JR, Ackland TR, Robertson WB, Fallon M, Zheng MH, Wood DJ. Knee Surg Sports Traumatol Arthrosc. 2014;22(1):2522-30.
Surgical Approach to Full Thickness Cartilage Defect With Patellar Instability: Combined Matrix-Associated Chondrocyte Implantation (MACI) and Medial Patellofemoral Ligament (MPFL) Reconstruction. Coladonato C, Wilson SM, Freedman KB. Video Journal of Sports Medicine. 2023;3(3).
A Case of Patellar Instability and Lateral Facet Cartilage Defect. Garcia GH, Haratian A, Hasan LK, et al. Video Journal of Sports Medicine. 2022;2(3).
Additional MACI publications
MACI Sandwich Technique With Autologous Bone Graft. Minas, T. (2024). Video Journal of Sports Medicine, 4(1), 26350254231188139.
Demonstration of the Medial Subvastus Knee Exposure for MACI Implantation. Jones, D. G., & Grilliot, J. M. (2023). Video Journal of Sports Medicine, 3(4).
Time Matters: Knee Cartilage Defect Expansion and High-Grade Lesion Formation while Awaiting Autologous Chondrocyte Implantation. Pettit RJ, Everhart JS, DiBartola AC, Blackwell RE, Flanigan DC. Cartilage. 2021;13(2):1802S-1808S.
Use of MACI (Autologous Cultured Chondrocytes on Porcine Collagen Membrane) in the United States: Preliminary Experience. Carey JL, Remmers AE, Flanigan DC. Orthopaedic Journal of Sports Medicine. 2020;8(8). Article first published online: August 12, 2020; Issue published: August 1, 2020.
Matrix-induced autologous chondrocyte implantation versus microfracture in the treatment of cartilage defects of the knee: a 2-year randomised study. Basad E, Ishaque B, Bachmann G, et al. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):519-27.
Clinical and radiological outcomes 5 years after matrix-induced autologous chondrocyte implantation in patients with symptomatic, traumatic chondral defects. Marlovits S, Aldrian S, Wondrasch B, Zak L, Albrecht C, Welsch G, Trattnig S. Am J Sports Med. 2012;40(10):2273-80.
Matrix-Associated Autologous Chondrocyte Implantation: A Clinical Follow-Up at 15 Years. Gille J, Behrens P, Schulz AP, Oheim R, Kienast B. Cartilage. 2016;(4):309-15.
Matrix-associated autologous chondrocyte transplantation/implantation (MACT/MACI)-5 year follow-up. Behrens P, Bitter T, Kurz B, Russlies M. The Knee. 2006;13(3):194-202.
MACI “Sandwich” Technique for a Large Osteochondritis Dissecans Lesion: A Case Report. Desai B, Jacobs G, Jones D. Case Reports in Orthopedics, vol. 2023, Article ID 7612206, 11 pages, 2023.
MACI (autologous cultured chondrocytes on porcine collagen membrane) in patients 40 years and older: short-term clinical outcomes and patient satisfaction. Leja L, Minas T. J Cart Joint Preserv, Articles in press 100102. Published: January 09, 2023.
Epidemiology, Progression and Morbidity of Cartilage Defects
Articular cartilage defects in 1,000 knee arthroscopies. Hjelle K, Solheim E, Strand T. Muri R, Brittberg M. Arthroscopy. 2002;18(7):730-4.
Focal Cartilage Defects in the Knee Impair Quality of Life as Much as Severe Osteoarthritis: A Comparison of Knee Injury and Osteoarthritis Outcome Score in 4 Patient Categories Scheduled for Knee Surgery. Heir S, Nerhus T, Ratterud J. Am J Sports Med. 2010;38(2):231-7.
Do focal chondral defects of the knee increase the risk for progression to osteoarthritis? A review of the literature. Houck D, Kraeutler MJ, Belk JW, Frank RM, McCarty EC, Bravman JT. Orthop J Sports Med. 2018;6(10) 2325967118801931. doi: 10.1177/2325967118801931
An overview of autologous chondrocyte implantation. Gikas PD, Bayliss L, Bentley G, Briggs TW. J Bone Joint Surg Br. 2009;91(8):997-1006.
Investigational Uses
Arthroscopic Delivery of Matrix-Induced Autologous Chondrocyte Implant: International Experience and Technique Recommendations. Cortese F, McNicholas M, Janes G, Gillogly S, Abelow SP, Gigante A, Coletti N. Cartilage. 2012 Apr;3(2):156-64.*
Arthroscopic matrix-induced autologous chondrocyte implantation: 2-year outcomes. Ebert JR, Fallon M, Ackland TR, Wood DJ, Janes GC. Arthroscopy. 2012 Jul;28(7):952-64.e1-2.*
*The implantation of MACI is currently approved to be performed via an arthrotomy to the knee joint. The arthroscopic delivery of MACI is an investigational use of the product and has not been approved in the United States.
A study of MACI in patients aged 10 to 17 years with symptomatic chondral or osteochondral defects of the knee: Design and rationale. Carey JL, Remmers AE, Ganley TJ. Pediatr Dimensions 6: DOI: 10.15761/PD.1000212.
Severe Burns
Epicel Journal Articles
CEA graft take after combining with a modified MEEK procedure. Craft-Coffman, B., Homsombath, B., Cramer, C., Hassan, Z., Fagan, S., Lack, K., & Wilson, J. (2024). Burns Open, 8(1), 23-28.
Report of outcomes in burn patients enrolled in the Cultured epidermal autograft prospective Registry. Fagan, S., Hassan, Z., Homsombath, B., Sood, R., Hardy, K., Craft-Coffman, B., Hartman, B. C., Cramer, C., & Griswold, J. (2024). Burns Open, 8(1), 29-34.
Use of Cultured Epithelial Autograft in Conjunction with Biodegradable Temporizing Matrix in Massive Burns: A Case Series. Heard, J., Sen, S., Greenhalgh, D., Palmieri, T., & Romanowski, K. (2023). J Burn Care Res, 44(6), 1434-1439.
Application and Management of Cultured Epidermal Autografts on Posterior Burns—A 5-Year, Multicenter, Retrospective Review of Outcomes. B. Homsombath, MD, R. Mullins, MD, C.Brandigi, MD, Z. Hassan, MD, S. Fagan, MD, B. Craft-Coffman, PA-C, Tait Olaveson, DO, P. Fidler, MD, C. Cramer, PhD, J. Hershman, MD. Journal of Burn Care & Research. 2022.
Twenty-Five Years’ Experience and Beyond with Cultured Epidermal Autografts for Coverage of Large Burn Wounds in Adult and Pediatric Patients, 1989-2015. W. Hickerson, MD, FACS, A. Remmers, PhD, DP Recker, MD. J Burn Care Res. 2019 Feb;40(2).
Cultured skin for massive burns: A prospective, controlled trial. Munster A. Reprinted from the Annals of Surgery. 1996;224(3):372-377.
Epicel Presentations and Posters
Cultured Epidermal Autografts (CEA) for Coverage of Large Burn Wounds in Pediatric and Adult Patients, 1989-2015. W. Hickerson, MD, FACS, T McKeen, J Fisher. 49th Annual Meeting of the American Burn Association (ABA), March 23, 2017.
NexoBrid Journal Articles
Early enzymatic burn debridement – results of the DETECT multicenter Randomized Controlled Trial. Y. Shoham, L. Rosenberg, W. Hickerson, J. Goverman, N. Iyer, J. Barrera-Oro, B. Lipovy, S. Monstrey, S. Blome-Eberwein, L. Wibbenmeyer, M. Scharpenberg, A. Singer, DETECT Investigators. Journal of Burn Care & Research. 2023.
Eschar removal by bromelain based enzymatic debridement (Nexobrid®) in burns: European consensus guidelines update. Hirche C, Kreken Almeland S, Dheansa B, Fuchs P, Governa M, Hoeksema H, Korzeniowski T, Lumenta DB, Marinescu S, Martinez-Mendez JR, Plock JA, Sander F, Ziegler B, Kneser U. Burns. 2020 Mar 30. pii: S0305-4179(19)30897-6. doi: 10.1016/j.burns.2020.03.002. [Epub ahead of print]
The enzymatic debridement for the treatment of burns of in-determinate depth. Bernagozzi F, Orlandi C, Purpura V, Morselli PG, Melandri D. J Burn Care Res. 2020 Mar 30. pii: iraa051. doi: 10.1093/jbcr/iraa051. [Epub ahead of print]
A novel rapid and selective enzymatic debridement agent for burn wound management: a multi-center RCT. Rosenberg L, Krieger Y, Bogdanov-Berezovski A, Silberstein E, Shoham Y, Singer AJ. Burns. 2014 May;40(3):466-74.
An Overview of the Use of Bromelain-Based Enzymatic Debridement (Nexobrid®) in Deep Partial and Full Thickness Burns: Appraising the Evidence. Loo YL, Goh BKL, Jeffery S. J Burn Care Res. 2018 Oct 23;39(6):932-938.
Time to start putting down the knife: A systematic review of burns excision tools of randomised and non-randomised trials.Edmondson SJ, Ali Jumabhoy I, Murray A. Burns. 2018 Nov;44(7):1721-1737.
Minimally invasive burn care: a review of seven clinical studies of rapid and selective debridement using a bromelain-based debriding enzyme (Nexobrid®). Rosenberg L, Shoham Y, Krieger Y, Rubin G, Sander F, Koller J, David K, Egosi D, Ahuja R, Singer AJ. Ann Burns Fire Disasters. 2015 Dec 31;28(4):264-274.
Enzymatic debridement for the treatment of severely burned upper extremities - early single center experiences. Cordts T, Horter J, Vogelpohl J, Kremer T, Kneser U, Hernekamp JF. BMC Dermatol. 2016 Jun 24;16(1):8.
The entity of thermal-crush-avulsion hand injury (hot-press roller burns) treated with fast acting debriding enzymes (nexobrid): literature review and report of first case. Di Castri A, Quarta L, Mataro I, Riccardi F, Pezone G, Giordano L, Shoham Y, Rosenberg L, Caleffi E. Ann Burns Fire Disasters. 2018 Mar 31;31(1):31.
Feasibility and safety of enzymatic debridement for the prevention of operative escharotomy in circumferential deep burns of the distal upper extremity. Fischer S, Haug V, Diehm Y, Rhodius P, Cordts T, Schmidt VJ, Kotsougiani D, Horter J, Kneser U, Hirche C. Surgery. 2019 Jan 21. pii: S0039-6060(18)30799-2.
Efficacy of enzymatic debridement of deeply burned hands. Krieger Y, Bogdanov-Berezovsky A, Gurfinkel R, Silberstein E, Sagi A, Rosenberg L. Burns. 2012 Feb;38(1):108-12.
Bromelain-based enzymatic debridement and minimal invasive modality (mim) care of deeply burned hands. Krieger Y, Rubin G, Schulz A, Rosenberg N, Levi A, Singer AJ, Rosenberg L, Shoham Y. Ann Burns Fire Disasters. 2017 Sep 30;30(3):198-204.
Selectivity of a bromelain based enzymatic debridement agent: A porcine study. Rosenberg L, Krieger Y, Silberstein E, Arnon O, Sinelnikov IA, Bogdanov-Berezovsky A, Singer AJ. Burns. 2012 Nov;38(7):1035-1040.
Enzymatic debridement of deeply burned faces: Healing and early scarring based on tissue preservation compared to traditional surgical debridement. Schulz A, Fuchs PC, Rothermundt I, Hoffmann A, Rosenberg L, Shoham Y, Oberländer H, Schiefer J. Burns. 2017 Sep;43(6):1233-1243.
The effects of rapid enzymatic debridement of deep partial-thickness burns with Debrase on wound reepithelialization in swine. Singer AJ, Taira BR, Anderson R, McClain SA, Rosenberg L. J Burn Care Res. 2010 Sep-Oct;31(5):795-802.
Reepithelialization of mid-dermal porcine burns after rapid enzymatic debridement with Debrase®. Singer AJ, Taira BR, Anderson R, McClain SA, Rosenberg L. J Burn Care Res. 2011 Nov-Dec;32(6):647-53.
Grants
Vericel is committed to improving the lives of patients with serious conditions by developing and manufacturing advanced cell therapies and specialty biologics. As part of this mission, Vericel is proud to support our orthopedic and burn surgeons and their patients through grants for research (investigator-sponsored trials), education, or donations to charitable entities.
Each grant request is evaluated on its individual merit. Please be advised that applications for grants may not be submitted via sales personnel, and sales personnel are not permitted to discuss pending applications. Submission of a grant proposal does not imply or guarantee approval. Please do not consider any request approved until you have received written documentation from Vericel.
Investigator-Sponsored Trials and Investigatory Initiated Studies
Vericel is committed to supporting investigator-sponsored trials (IST) for the advancement of scientific and medical knowledge of our products. Each IST is carefully evaluated on a competitive basis, taking into account scientific rigor, methodological considerations, and patient safety.
To submit an application: download and complete the Application for Investigator Sponsored Trial and then click the “Email” button at the top which will automatically generate an email to [email protected]
Education Grants
Vericel may provide financial support for valid healthcare professional educational activities organized by legitimate third-party organizations with educational missions.
To submit an application: download and complete the Application for Educational Grant and then click the “Email” button at the top which will automatically generate an email to [email protected]
Charitable Donations
To support a recipient’s legitimate charitable mission, Vericel may provide charitable donations in the form of financial or in-kind support to qualified 501(c)(3) nonprofits or similar charitable organizations.
To submit an application: please send a letter of request to [email protected]
NASDAQ: VCEL
| $46.05 | 0.6 (1.32%) |
Day Low: $45.4
Volume: 284638
April 26, 2024